Cannabis cosmetics were once considered the next frontier in the skin care industry. However, following a notice issued by the Chinese People's Procuratorate, the marijuana skin care craze was immediately suspended.
On March 26, the China Institute for Food and Drug Control issued the "Notice of the China Inspection Institute on Public Consultation on Revising Prohibited Components of Cosmetics" (hereinafter referred to as the notice). According to the "Notice", according to the relevant national drug control policy requirements, it is proposed to list raw materials such as cannabis (CANNABISSATIVA) nuts, cannabis (CANNABIS SATIVA) seed oil, cannabis (CANNABIS SATIVA) leaf extract and cannabidiol (cannabidiol) as cosmetics The banned components are now open to the public for comments.
As soon as the news came out, industry insiders called out: hemp concept cosmetics are going to be cold!
CBD cosmetics chaos
Hemp conceptual cosmetics have been a hot spot in the skin care industry in the past two years. Hemp cosmetics refer to cosmetics that are added with raw materials from industrial hemp. These raw materials include hemp seed oil, hemp seeds, hemp leaf extract, cannabidiol (CBD), etc.
In my country’s current "List of Names of Used Cosmetic Ingredients" (2015 edition), hemp seeds, hemp seed oil, and hemp leaf extract related ingredients are included as legally used ingredients, while cannabidiol (CBD) is not listed. Into it.
The announcement of the "Notice" means that raw materials including cannabidiol (CBD) may be regulated as prohibited ingredients.
Global Web Health searched on an e-commerce platform and found that cosmetic products with CBD ingredients were still on sale, and some companies even marked CBD on their product packaging.
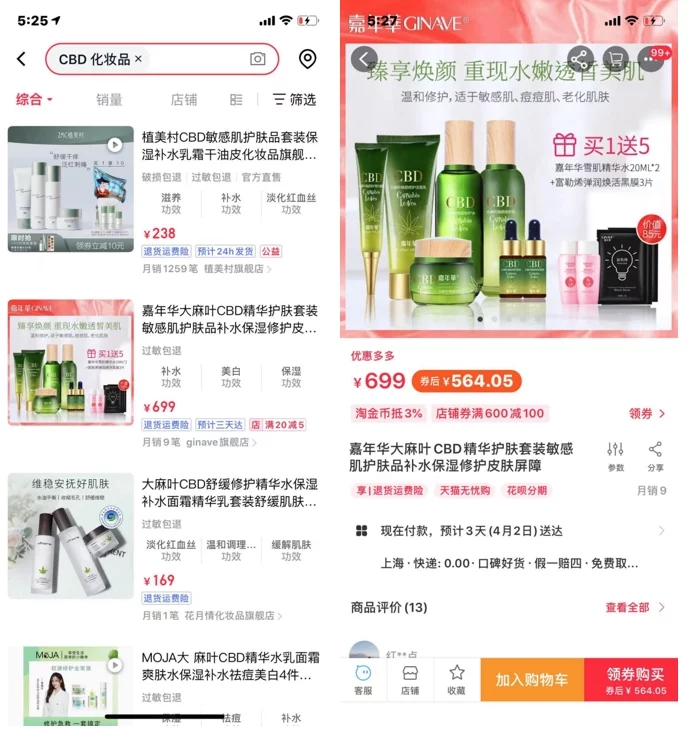
Industry insiders believe that in this "Notice", hemp seeds, hemp seed oil, and hemp leaf extracts that were originally compliant are also included in the list of ingredients to be banned. Once the ban comes true, hemp cosmetics will face " Blocked across the board".
Where will hemp conceptual cosmetics go?
In recent years, cannabidiol (CBD) has become the most emerging and popular ingredient in foreign health care products and skin care products. The development of various CBD skin care products is in full swing. This CBD "skin care wind" has also blown domestically. Many drugs Enterprises, capital, cosmetics companies and brands have entered the game. According to statistics, in 2020 alone, 1783 cannabis-related filings were added to product filings on cannabis ingredients, which is four times the number in 2019.
It is understood that many international brands such as Origins, Avon and The Body Shop under Estee Lauder have already launched CBD products. In China, brands such as Man Ting, Uemimura, Yi Ye Zi, and Chun Ji have launched cannabis ingredient products. At the same time, the CBD fever also gave birth to brands such as the new miracle of CANNA FEVER, hemp makeup, and Xi Muyuan.
Since the "Regulations on the Supervision and Administration of Cosmetics" was formally implemented, the country's regulation of cosmetics has become stricter and standardized, and it has become an industry consensus. The "Notice" mentioned in "according to the relevant national drug control policy requirements" also reminds the country to tighten the control of cannabis-related products and ingredients.
It is worth noting that recently, Tmall International issued a notice on the update of the "Tmall International Cosmetics Platform Standard" (hereinafter referred to as the announcement) to strengthen the management of the cosmetics industry. The announcement showed that after the change, the product attribute release specification was adjusted to correctly indicate the brand, product specifications, product name, country of origin, cosmetic shelf life, deadline use date range, cosmetic net content, usage method, warnings, and whether it contains or not. CBD etc. Compared with the product attribute release specifications before the change, there is an additional item of "Does it contain CBD". When you select "Yes", you need to upload the tetrahydrocannabinol (THC) test report of the corresponding product, and the THC content should not exceed 0.3%.
Many signals indicate that cannabis cosmetics will face a "life and death" test, where will it go? We will wait and see.


